Abstracts 2010
Vittoria Blasucci, Ryan Hart, Veronica Llopis Mestre, Dominique Julia Hahne, Melissa Burlager, Hillary Huttenhower, Beng Joo Reginald Thio, Charles L. Liotta, and Charles A. Eckert, “Single Component, Reversible Ionic Liquids for Energy Applications,” Fuel, 89, 1315–1319, 2010.
Single component, reversible ionic liquids have excellent potential as novel solvents for a variety of energy applications. Our energy industry is faced with many new challenges including increased energy consumption, depleting oil reserves, and increased environmental awareness. We report the use of reversible ionic liquids to solve two energy challenges: extraction of hydrocarbons from contaminated crude oil and carbon capture from power plant flue gas streams. Our reversible solvents are derived from silylated amine molecular liquids which react with carbon dioxide reversibly to form ionic liquids. Here we compare the properties of various silylated amine precursors and their corresponding ionic liquids. We show how the property changes are advantageous in the two aforementioned energy applications. In the case of hydrocarbon purification, we take advantage of the polarity switch between precursor and ionic liquid to enable separations. In carbon capture, our solvents act as dual physical and chemical capture agents for carbon dioxide. Finally, we show the potential economics of scale-up for both processes.
Vittoria M. Blasucci, Zainul A. Husain, Ali Z. Fadhel, Megan E. Donaldson, Eduardo Vyhmeister, Pamela Pollet, Charles L. Liotta*, and Charles A. Eckert, “Combining Homogeneous Catalysis with Heterogeneous Separation using Tunable Solvent Systems,” J. Phys. Chem. A, 2010, 114, 3932–3938.
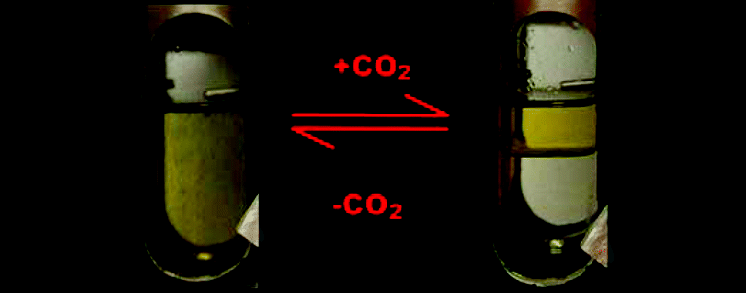 |
Tunable solvent systems couple homogeneous catalytic reactions to heterogeneous separations, thereby combining multiple unit operations into a single step and subsequently reducing waste generation and improving process economics. In addition, tunable solvents can require less energy than traditional separations, such as distillation. We extend the impact of such solvents by reporting on the application of two previously described carbon dioxide tunable solvent systems: polyethylene glycol (PEG)/organic tunable solvents (POTS) and organic/aqueous tunable solvents (OATS). In particular, we studied: (1) the palladium catalyzed carbon−oxygen coupling of 1-bromo-3,5-dimethylbenzene and o-cresol to potassium hydroxide to produce o-tolyl-3,5-xylyl ether and 1-bromo-3,5-di-tert-butylbenzene to potassium hydroxide to produce 3,5-di-tert-butylphenol in PEG400/1,4-dioxane/water and (2) the rhodium-catalyzed hydroformylation of p-methylstyrene in water/acetonitrile to form 2-(p-tolyl) propanal. In addition, we introduce a novel tunable solvent system based on a modified OATS where propane replaces carbon dioxide. This represents the first use of propane in a tunable solvent system.
Ryan Hart, Pamela Pollet, Dominique J. Hahne, Ejae John, Veronica Llopis-Mestre, Vittoria Blasucci, Hillary Huttenhower, Walter Leitner, Charles A. Eckert, Charles L. Liotta, “Benign Coupling of Reactions and Separations with Reversible Ionic Liquids,” Tetrahedron, 66, 1082–1090, 2010.
Reversible ionic liquids are a novel class of solvents that combine an effective medium where reactions occur with a ‘built-in’ separation ability for facile recovery of the products and catalysts, making the solvent available for recycle. We report the utility of these solvents in a number of reactions (Claisen–Schmidt condensation, Heck C–C coupling, and CO2 capture) and discuss the effectiveness of the separation. We also provide insight into the challenges and limitations of using these unique solvent systems to couple reactions and separations.
Vittoria M. Blasucci, Ryan Hart, Pamela Pollet, Charles L. Liotta, and Charles A. Eckert, “Reversible Ionic Liquids Designed for Facile Separations,” Fluid Phase Equilibria, 294, 1 – 6, 2010.
Reversible ionic liquids can increase the application of traditional ionic liquids, such as those based on
imidazolium cations, and generate new technologies along the way. While traditional ionic liquids have
gained industrial and academic attention as solvents, many applications suffer from the difficulty associated
with removing high boiling point products from non-volatile liquids. Reversible ionic liquids are
those which can be reversed back and forth between molecular and ionic forms enabling facile separations
through large in situ property changes. Thus these novel solvents lead to truly sustainable processes.
An understanding of the phase behavior of these systems provides insight into the separation capability. Here we review both one- and two-component reversible ionic liquids and their applications with an emphasis on separations.
Pamela Pollet, Ryan J. Hart, Charles A. Eckert, Charles L. Liotta, “Organic Aqueous Tunable Solvents (OATS): A Vehicle for Coupling Reactions and Separations,” Accounts of Chemical Research, 43 (9), 1237-1245, 2010.
In laboratory-based chemical synthesis, the choice of the solvent and the means of product purification are rarely determined by cost or environmental impact considerations. When a reaction is scaled up for industrial applications, however, these choices are critical: the separation of product from the solvent, starting materials, and byproduct usually constitutes 60-80% of the overall cost of a process. In response, researchers have developed solvents and solvent-handling methods to optimize both the reaction
and the subsequent separation steps on the manufacturing scale. These include “switchable” solvents, which are designed so that their physical properties can be changed abruptly, as well as “tunable” solvents, wherein the solvent’s properties change continuously through the application of an external stimulus. In this Account, we describe the organic aqueous tunable solvent (OATS) system, examining two instructive and successful areas of application of OATS as well as its clear potential for further refinement.
OATS systems address the limitations of biphasic processes by optimizing reactions and separations simultaneously. The reaction is performed homogeneously in a miscible aqueous-organic solvent mixture, such as water-tetrahydrofuran (THF). The efficient product separation is conducted heterogeneously by the simple addition of modest pressures of CO2 (50-60 bar) to the
system. Under these conditions, the water-THF phase splits into two relatively immiscible phases: the organic THF phase contains the hydrophobic product, and the aqueous phase contains the hydrophilic catalyst. We take advantage of the unique properties of OATS to develop environmentally benign and cost-competitive processes relevant in industrial applications. Specifically, we describe the use of OATS for optimizing the reaction, separation, design, and recycling of (i) Rh-catalyzed hydroformylation of olefins such as 1-octene and (ii) enzyme-catalyzed hydrolysis of 2-phenylacetate.
We discuss the advantages of these OATS systems over more traditional processes. We also consider future directions that can be taken with these proven systems as well as related innovations that have recently been reported, including the use of poly-(ethylene glycol) (PEG) as a tunable adjunct in the solvent and the substitution of propane for CO2 as the external stimulus. OATS systems in fact represent the ultimate goal for a sustainable process, because in an idealized setup there is only reactant coming in and product going out; in principle, there is no waste stream.
Juncheng Liu, Nicholas Ruffini, Pamela Pollet, Veronica Llopis-Mestre, Cerag Dilek, Charles A. Eckert, Charles L. Liotta, Christopher B. Roberts, "More Benign Synthesis of Palladium Nanoparticles in Dimethyl Sulfoxide and Their Extraction into an Organic Phase," I&EC Res, 49 (17), 8174-8179, 2010.
We present the successful synthesis and stabilization of 3.5 nm Pd nanoparticles (standard deviation of 0.49 nm) within dimethyl sulfoxide (DMSO) via fast, homogeneous reduction of a Pd salt using NaBH4 in the
absence of traditional capping ligands. These Pd nanoparticles were found to be extremely stable and did not
exhibit precipitation and/or agglomeration within the DMSO solvent even after more than 9 months. Moreover,
these Pd nanoparticles were conveniently separated from the DMSO solvent medium via vacuum freeze drying
by taking advantage of the high freezing point of DMSO. We have also successfully extracted the Pd nanoparticles from the DMSO phase into an organic phase (i.e., hexane), thereby providing a facile and
efficient means for the generation of organic phase dispersible metal nanoparticles with complete recycle of the DMSO solvent.
Gregory A. Marus, Eduardo Vyhmeister, Pamela Pollet, Megan E. Donaldson, Veronica Llopis-Mestre, Sean Faltermeier, Renee Roesel, Leslie Gelbaum, Charles L. Liotta, and Charles A. Eckert, “The Sustainable and Scalable Synthesis of Piperylene Sulfone: A “Volatile” and Recyclable DMSO Substitute,” I&EC Res, in press, 2010.
An essential feature of chemical research is advancing new laboratory findings to a form useful to industry. Dimethyl sulfoxide (DMSO) is an important dipolar, aprotic solvent for conducting chemical reactions. Unfortunately, separation of the products of reaction from the solvent is difficult and expensive. We have proposed the use of piperylene sulfone (PS) as a substitute for DMSO, and herein, we establish a roadmap to its sustainable and scalable synthesis. PS is a potentially important new dipolar aprotic solvent that has solvent properties similar to those of DMSO; in contrast to DMSO, PS is fully recyclable and undergoes a reversible retrocheletropic reaction at 110 °C, permitting facile solvent removal and recycle. PS is synthesized by the reaction of trans-piperylene with sulfur dioxide. Because PS is not commercially available, we
synthesized laboratory quantities using a method not sustainable on a large scale because of expensive chemicals
and considerable waste generation. To develop and optimize a scalable process, we (1) determined the kinetic
parameters associated with the reaction by employing in situ proton NMR measurements and (2) studied the
effects of radical inhibitors in reducing unwanted side reactions. In addition, we recovered PS from the reaction mixture through a sustainable CO2 separation method, which resulted in a substantial waste reduction. Our development of a more efficient, safe, and sustainable scaleup method for PS thus illustrates an important aspect of chemical research: the need to render the results usable and useful to industry.
Ali Z. Fadhel, Pamela Pollet, Charles L. Liotta, and Charles A. Eckert, “Combining the Benefits of Homogeneous and Heterogeneous Catalysis with Tunable Solvents and Nearcritcal Water,” Molecules, 15, 8400-8424, 2010.
The greatest advantage of heterogeneous catalysis is the ease of separation,
while the disadvantages are often limited activity and selectivity. We report solvents that
use tunable phase behavior to achieve homogeneous catalysis with ease of separation.
Tunable solvents are homogeneous mixtures of water or polyethylene glycol with organics
such as acetonitrile, dioxane, and THF that can be used for homogeneously catalyzed
reactions. Modest pressures of a soluble gas, generally CO2, achieve facile post-reaction
heterogeneous separation of products from the catalyst. Examples shown here are rhodiumcatalyzed
hydroformylation of 1-octene and p-methylstyrene and palladium catalyzed C-O
coupling to produce o-tolyl-3,5-xylyl ether and 3,5-di-tert-butylphenol. Both were
successfully carried out in homogeneous tunable solvents followed by separation
efficiencies of up to 99% with CO2 pressures of 3 MPa. Further examples in tunable
solvents are enzyme catalyzed reactions such as kinetic resolution of rac-1-phenylethyl
acetate and hydrolysis of 2-phenylethyl acetate (2PEA) to 2-phenylethanol (2PE). Another
tunable solvent is nearcritical water (NCW), whose unique properties offer advantages for
developing sustainable alternatives to traditional processes. Some examples discussed are Friedel-Crafts alkylation and acylation, hydrolysis of benzoate esters, and water-catalyzed deprotection of N-Boc-protected amine compounds.
|
